Chemical Engineering Mock Interview 3
Mock interview questions covering mass transfer, mathematical analysis, and phase equilibria
3 questions • Estimated time: 45-60 minutes
How to Use This Mock
- Read each question carefully
- Attempt your own answer first — spend at least 5 minutes thinking
- Only reveal the model answer after you've tried
- Compare your reasoning to the model answer
Nitrogen is bubbled through a drum containing liquid n-hexane at a rate of 0.1 kmol/min. The gas stream leaving the drum contains 10.0 mol% n-hexane vapour.
Estimate the time required to vaporise 0.1 m³ of liquid n-hexane. Assume that nitrogen is insoluble in liquid n-hexane.
Data: The density of liquid n-hexane is kg/m³.
Model Answer
Initial hypothesis:
The total molar flow rate leaving the drum is 0.1 kmol/min of nitrogen plus the hexane that has been vaporised. So that is .
You said that the gas stream leaving the drum contains 10.0 mol% n-hexane vapour, so
Working through:
Solving for :
Interviewer follow-up:
Good. Now how many moles of hexane do you have initially in 0.1 m³?
Student responds:
I need the molecular weight. n-hexane is C₆H₁₄, so kg/kmol. The mass is:
Interviewer:
Perfect. Now you can find the time.
Final answer:
Sanity check:
Just over an hour to strip a small drum at this flowrate seems reasonable.
Sketch the function .
Consider: How the frequency of oscillation changes with , behaviour at the origin, and approximate locations of maxima and minima.
Model Answer
So, it is going to be some sort of variation on the graph of .
It will wiggle with an amplitude of 1 unit.
I know that grows faster than , so that means the frequency of zero crossings and maxima will increase as we move further from the origin according to .
Now what is going to happen close to zero...
Well for small I know , so I assume that and that lets me work out the shape close to zero
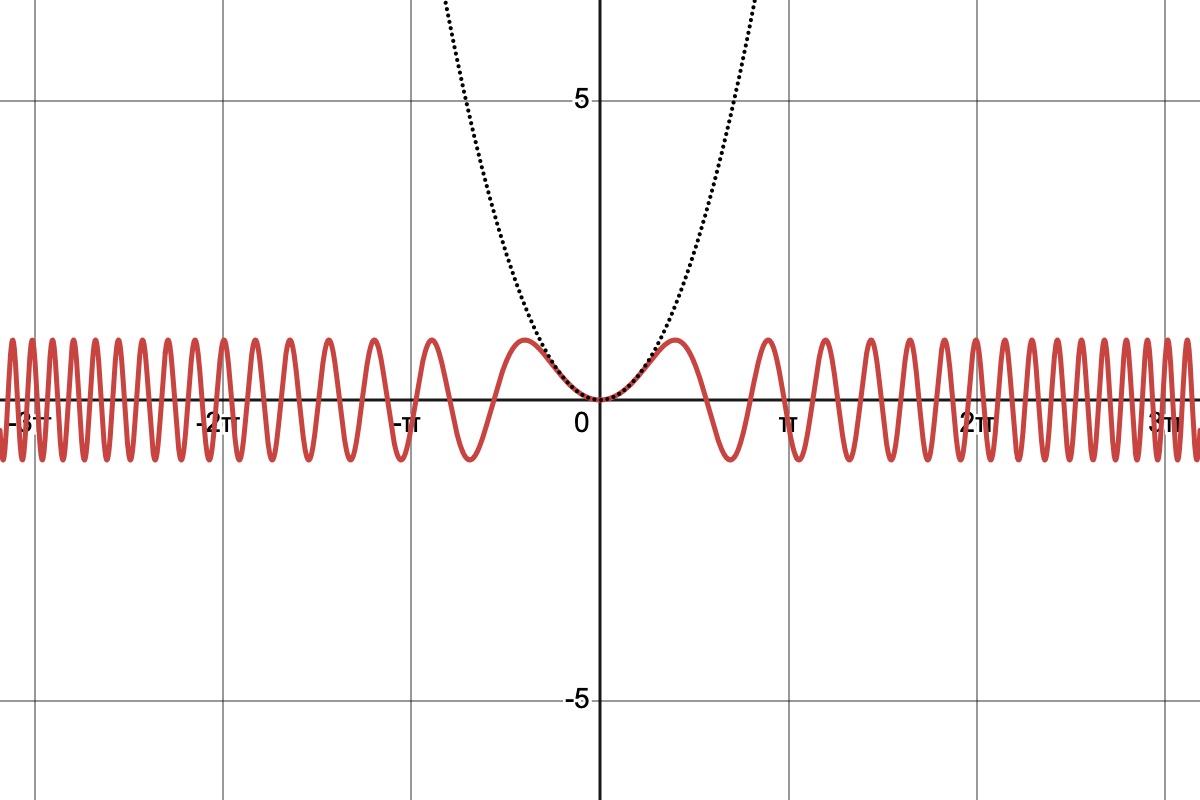
Click to enlarge
A puddle of water left on the ground will gradually disappear, even though its temperature is well below the boiling point of water.
How would you explain this observation in terms of molecular behaviour?
Model Answer
Initial hypothesis:
The water molecules are escaping into the air somehow?
Interviewer Nudge:
Yes, but why can they escape if the temperature is below boiling point? Think about molecular kinetic energies.
Refined response:
Not all molecules have the same kinetic energy—there's a distribution. Some molecules have much higher energy than the average, following the Maxwell-Boltzmann distribution.
Interviewer follow-up:
Good. So what happens when a high-energy molecule reaches the liquid surface?
Student continues:
If it has enough energy to overcome the intermolecular forces, it can escape into the gas phase. This is evaporation. At boiling point, molecules throughout the bulk can escape, but below it, only high-energy surface molecules evaporate.
Interviewer:
Exactly. But there's also condensation—water molecules in the air can return to the liquid. Why does the puddle eventually disappear?
Final answer:
It's a balance between evaporation and condensation rates. If the air is dry (low humidity), evaporation exceeds condensation, so the puddle shrinks. The rate depends on temperature (faster molecular motion), surface area, air flow (wind removes water vapour), and humidity (lower humidity increases net evaporation). In the open air, the puddle eventually disappears completely.