Chemical Engineering Mock Interview
Mock interview questions covering fluid statics, mathematical analysis, and engineering estimation
6 questions • Estimated time: 45-60 minutes
How to Use This Mock
- Read each question carefully
- Attempt your own answer first — spend at least 5 minutes thinking
- Only reveal the model answer after you've tried
- Compare your reasoning to the model answer
Let's think about how air pressure changes as we go higher in the atmosphere. We know that in any fluid at rest under gravity, the pressure gradient is given by:
Part (a): Assuming that the atmosphere has a constant temperature, , how do you expect to vary with ?
Model Answer
Initial hypothesis:
I think we just need to integrate that equation?
Interviewer Nudge:
That's the right instinct, how can we handle the fact that can vary with height?
Refined response:
Oh, I see! Since air is a gas, we could use the ideal gas law:
So in the end I get:
Part (b): Your expression for introduces a characteristic vertical lengthscale. What does this tell you about why we don't notice pressure changes when standing up?
Model Answer
Initial hypothesis:
Is it just... ?
Interviewer Nudge:
Yes, exactly. This is called the scale height. Now let's get physical intuition: what does this length represent, and can you estimate its value?
Refined response:
Let me see... kg/mol, J/(mol·K), K, m/s². So
Interviewer follow-up:
Excellent calculation. So the pressure changes by a factor of about for every 10 km you go up.
Final answer:
So if you stand up, you only move about a meter, that's 1/10,000 of the characteristic lengthscale. That's why we don't feel lightheaded or notice any difference when standing up from pressure changes alone.
Part (c): The assumption of constant temperature is quite poor. In reality, temperature typically decreases with height. Suppose instead:
where . How does this modify the relationship between density and pressure?
Model Answer
Initial hypothesis:
We'd just... substitute this temperature into the ideal gas law?
Interviewer Nudge:
Yes, good start. Walk me through it.
Refined response:
From before, . But now , so
So
which gives us:
Part (d): Can you show that letting recovers your earlier result?
Model Answer
One definition of the exponential is
Letting , as so
Interviewer conclusion:
Perfect. And why is this kind of limiting check so valuable in physics and engineering?
Student reflection:
It shows our more complex model correctly simplifies to the simpler case we already understood, which gives us confidence we haven't made errors.
Sketch the function:
Model Answer
Okay, so I can see this graph is going to have two types of limiting behaviour: close to the origin it will look like and far away it will look like , so let me just sketch those curves (dotted lines).
Next I'll have a look for solutions to and I can see that there will only be one at
In terms of turning points, I can differentiate it and set that equal to zero
Putting those together I can join the dots to make my sketch
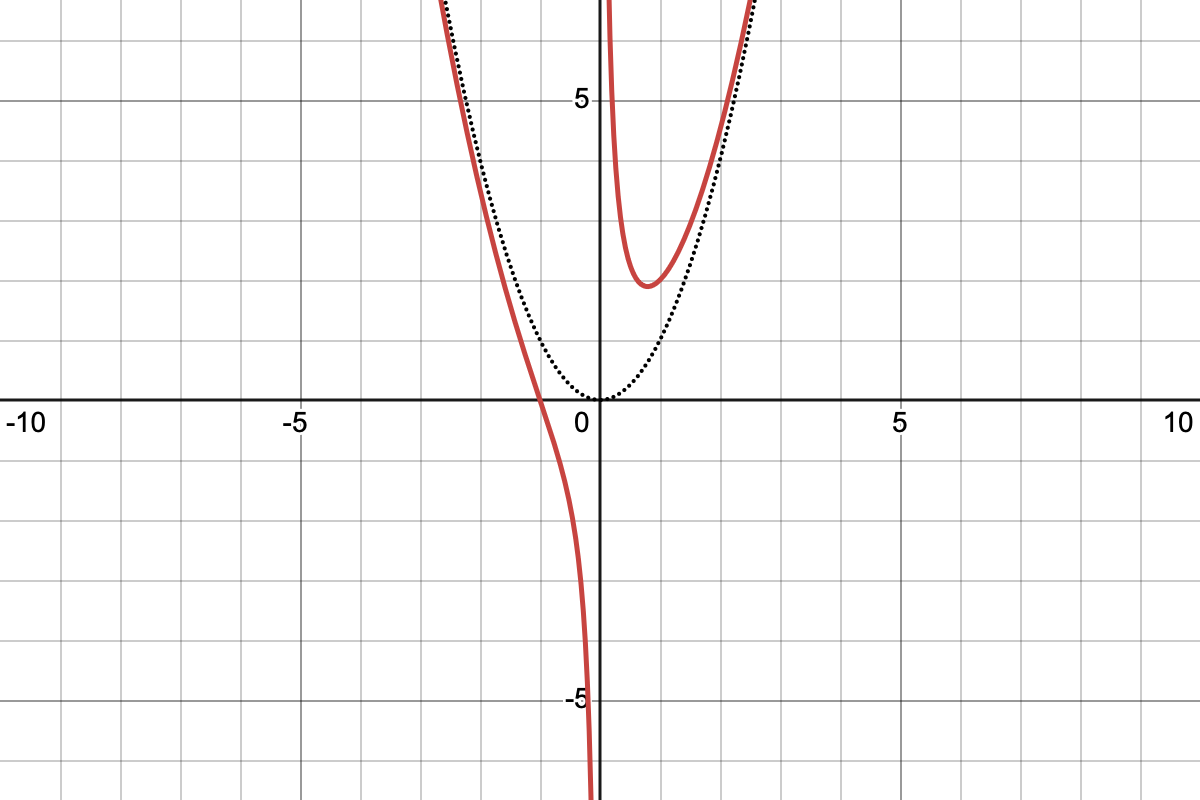
Click to enlarge
A chemical plant has a reactor releasing 100 MW of heat continuously. We need to remove this heat using cooling water. Estimate how much water flow we'd need.
Model Answer
Initial hypothesis:
Maybe thousands of liters per second?
Interviewer Nudge:
Let's work systematically. What equation relates heat transfer rate, mass flow rate, and temperature change?
Refined response:
where is heat rate, is mass flow, is specific heat capacity, and is temperature rise.
Interviewer follow-up:
Good. Now estimate: what's the specific heat capacity of water, and what's a reasonable for industrial cooling?
Student responds:
J/(kg·K). For , maybe 10°C to avoid scaling and approaching boiling.
Calculation:
So: Converting to volumetric flow:
Sanity check:
This is equivalent to about 14,000 garden hoses—huge, but reasonable for a 100 MW industrial plant with cooling towers or river access.